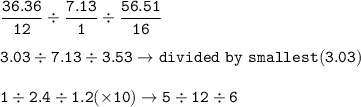Its empirical formula : C₅H₁₂O₆
Further explanation
The empirical formula is the smallest comparison of atoms of compound forming elements.
(empirical formula) n = molecular formula
The principle of determining empirical formula and molecular formula
- Determine the mass ratio of the constituent elements of the compound.
- Determine the mole ratio by dividing the elemental mass with the relative atomic mass obtained by the empirical formula
Ar C = 12 g/mol
Ar H = 1 g/mol
Ar O = 16 g/mol
mol ratio of C : H : O =