Hey There!
_____________________________________
Answer:
_____________________________________
ATOMIC NUMBER: It is the number of protons present in the nucleus of every atom
MASS NUMBER: It is the number of nucleon. Nucleon are the number of proton and neutrons present in the nucleus.
PROTON NUMBER: Proton number equal to the atomic number.
ELECTRON NUMBER: Electron Number is equal to the atomic number.
NEUTRON NUMBER: It is Mass Number - Atomic Number.
CHARGE: It is due to the addition or removal of the electron. + charge when electron removed and - charge when electron is added.
SYMBOL: It is represented as,
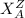
Z is Mass Number
A is Atomic Number
_____________________________________
Worksheet:
(I) ATOMIC NUMBER: 17
MASS NUMBER: 35.5
PROTON NUMBER: 17
ELECTRON NUMBER: 17
NEUTRON NUMBER: 18.5
CHARGE: 0
_____________________________________
(II) ATOMIC NUMBER: 71
MASS NUMBER: 180
PROTON NUMBER: 71
ELECTRON NUMBER: 71
NEUTRON NUMBER: 109
CHARGE: 0
_____________________________________
(III) ATOMIC NUMBER: 40
MASS NUMBER: 86
PROTON NUMBER: 40
ELECTRON NUMBER: 38
NEUTRON NUMBER: 46
CHARGE: +2
_____________________________________
(IV) ATOMIC NUMBER: 92
MASS NUMBER: 238
PROTON NUMBER: 92
ELECTRON NUMBER: 86
NEUTRON NUMBER: 146
CHARGE: +6
_____________________________________
(V) ATOMIC NUMBER: 82
MASS NUMBER: 206
PROTON NUMBER: 82
ELECTRON NUMBER: 78
NEUTRON NUMBER: 124
CHARGE: +4
_____________________________________
(VI) ATOMIC NUMBER: 34
MASS NUMBER: 79
PROTON NUMBER: 34
ELECTRON NUMBER: 36
NEUTRON NUMBER: 45
CHARGE: -2
_____________________________________
(VII) ATOMIC NUMBER: 48
MASS NUMBER: 113
PROTON NUMBER: 48
ELECTRON NUMBER: 49
NEUTRON NUMBER: 65
CHARGE: -1
_____________________________________
(VIII) ATOMIC NUMBER: 21
MASS NUMBER: 42
PROTON NUMBER: 21
ELECTRON NUMBER: 21
NEUTRON NUMBER: 21
CHARGE: 0
_____________________________________
(IX) ATOMIC NUMBER:
MASS NUMBER:
PROTON NUMBER:
ELECTRON NUMBER:
NEUTRON NUMBER:
CHARGE:
_____________________________________
(X) ATOMIC NUMBER: 83
MASS NUMBER: 209
PROTON NUMBER: 83
ELECTRON NUMBER: 80
NEUTRON NUMBER: 126
CHARGE: +3
_____________________________________
(XI) ATOMIC NUMBER: 47
MASS NUMBER: 108
PROTON NUMBER: 47
ELECTRON NUMBER: 47
NEUTRON NUMBER: 61
CHARGE: 0
_____________________________________
(XII) ATOMIC NUMBER: 49
MASS NUMBER: 116
PROTON NUMBER: 49
ELECTRON NUMBER: 46
NEUTRON NUMBER: 67
CHARGE: +3
_____________________________________
(XIII) ATOMIC NUMBER: 53
MASS NUMBER: 128
PROTON NUMBER: 53
ELECTRON NUMBER: 54
NEUTRON NUMBER: 75
CHARGE: -1
_____________________________________
(XIV) ATOMIC NUMBER: 76
MASS NUMBER: 188
PROTON NUMBER: 76
ELECTRON NUMBER: 72
NEUTRON NUMBER: 112
CHARGE: +4
_____________________________________
Best Regards,
'Borz'