Answer:
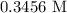
Step-by-step explanation:
 = Volume of NaOH = 34.56 mL
= Volume of NaOH = 34.56 mL
 = Volume of HCl = 25 mL
= Volume of HCl = 25 mL
 = Concentration of NaOH = 0.25 M
= Concentration of NaOH = 0.25 M
 = Concentration of HCl
= Concentration of HCl
When endpoint is reached the number of moles of NaOH will be equal to the number of moles of HCl
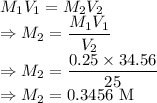
Concentration of HCl is
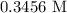 .
.