Answer:
Metal is copper.
Step-by-step explanation:
Mass of a metal, m = 54 g
Heat energy required, Q = 773 J
The temperature changes from 63.0 °C to 100.0 °C.
We need to identify the metal. Heat required to raise the temperature is given by :
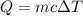
c is specific heat of the metal
We can identify the metal by finding its specific heat.
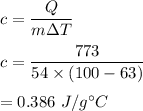
The metal is copper. Its specific heat is
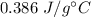 .
.