Answer: The volume of this sample at STP is 15.4 L
Step-by-step explanation:
The combined gas equation is,
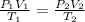
where,
 = initial pressure of gas = 210 kPa
= initial pressure of gas = 210 kPa
 = final pressure of gas = 101.3 kPa ( at STP)
= final pressure of gas = 101.3 kPa ( at STP)
 = initial volume of gas = 9.50 L
= initial volume of gas = 9.50 L
 = final volume of gas = ? ( at STP)
= final volume of gas = ? ( at STP)
 = initial temperature of gas = 350 K
= initial temperature of gas = 350 K
 = final temperature of gas = 273 K ( at STP)
= final temperature of gas = 273 K ( at STP)
Now put all the given values in the above equation, we get:
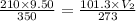
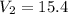
The volume of this sample at STP is 15.4 L