Write 0.8 as 0.8/1.
Multiply the numerator and denominator by 10 for each digit after the decimal point:
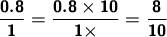
To reduce the fraction, we find the greatest common factor (GCF) for 8 and 10. A factor is just a number that is divided into another number without any remainder.
- The factors of 8 are: 1,2,4,8
- The factors of 10 are: 1, 2, 5, 10
The greatest common factor (GCF) for both 8 and 10 is: 2.
Now, to reduce the fraction, we divide both the numerator and denominator by the value of the GCF.
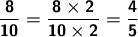
As a side note, the integral part of the integer is: empty
The decimal part is: 8 = ⁸/₁₀
Complete breakdown of simple fractions: 80/100
= 8/10
= 4/5
