Answer:
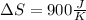
Step-by-step explanation:
Hello!
In this case, since the information is not missing, the following one will be used as it is found on similar problems:

In such a way, we use the following equation to compute the entropy:
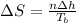
Whereas n (moles) are computed by using its molar mass (74.14 g/mol) as follows:
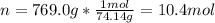
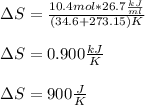
Best regards!