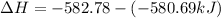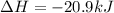Answer: Thus
 for the conversion of gray tin to white tin is -20.9 kJ
for the conversion of gray tin to white tin is -20.9 kJ
Step-by-step explanation:
According to Hess’s law of constant heat summation, the heat absorbed or evolved in a given chemical equation is the same whether the process occurs in one step or several steps.
According to this law, enthalpy change of the overall reaction is the sum of the enthalpy changes of the intermediate reactions.
The final reaction is
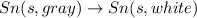
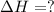
The intermediate balanced chemical reaction will be,
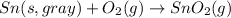
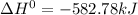 (1)
(1)
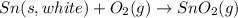
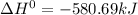 (2)
(2)
substarcting (2) form (1) we get