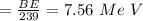Answer:
The answer is "
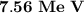 ".
".
Step-by-step explanation:
calculating the binding energy on per nucleon:
calculating number of proton and neutrons:
proton
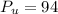
neutron
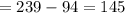
calculating mass:
proton mass
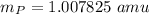
neutron mass
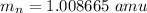
neutral atom mass
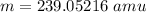
mass of prtons
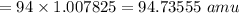
mass of neutrons
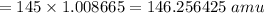
Total nucleons mass formula:
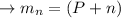
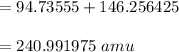
calculating the mass of defect:
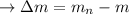
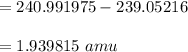
calculating the total of the binding energy:
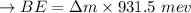
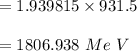
BE in per nucleon