Answer:
The correct option is C: the isotope of iodine contains 81 neutrons.
Explanation:
The mass (A) of the iodine isotope is given by:
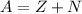
Where:
Z: is the number of protons
N: is the number of neutrons
For iodine Z= 53, so if A = 134 the number of neutrons is:
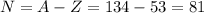
Therefore, the correct option is C. The isotope of iodine contains 81 neutrons.
I hope it helps you!