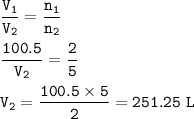The volume of Oxygen at STP required : 251.25 L
Further explanation
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters/mol.
Reaction(combustion) :
2C₂H₂(g)+5O₂(g)⇒4CO₂(g)+2H₂O(g)
mol C₂H₂ :
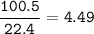
mol ratio C₂H₂ : O₂ = 2 : 5, so mol O₂ :
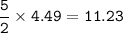
volume O₂ at STP :
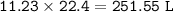
Or you can use Avogadro's hypothesis :
In the same temperature and pressure, in the same volume conditions, the gas contains the same number of molecules
So the ratio of gas volume will be equal to the ratio of gas moles :