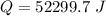Answer:
First question
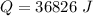
Second question
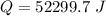
Step-by-step explanation:
From the question we are told that
The melting point of Ethanol is
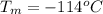
The boiling point of Ethanol is
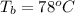
The enthalpy of fusion of Ethanol is
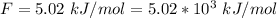
The enthalpy of vaporization of Ethanol is
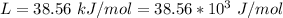
The specific heat of solid Ethanol is
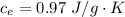
The specific heat of liquid Ethanol is
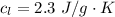
The mass of the Ethanol given is
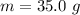
Considering the first question
The initial temperature is
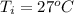
The final temperature is
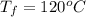
Generally the heat required too raise the Ethanol to its boiling point is mathematically represented as
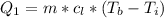
=>
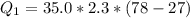
=>
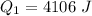
Genially the number of moles of Ethanol given is mathematically represented as
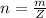
Here Z is the molar mass of Ethanol with value
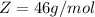
So
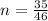
=>
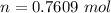
Generally the heat of vaporization of the Ethanol is mathematically represented as
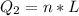
=>
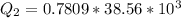
=>
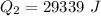
Generally the heat required too raise the Ethanol from its boiling point to
 is mathematically represented as
is mathematically represented as
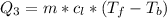
=>
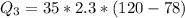
=>
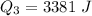
Generally the total heat required is
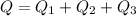
=>
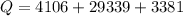
=>
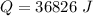
Considering the second question
The initial temperature is
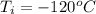
The final temperature is
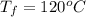
Generally the heat required too raise the Ethanol to its melting point is mathematically represented as
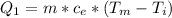
=>
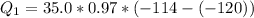
=>
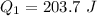
Generally the heat of fusion of the Ethanol is mathematically represented as
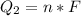
=>
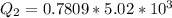
=>
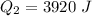
Generally the heat required too raise the Ethanol to its boiling point is mathematically represented as
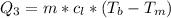
=>
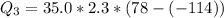
=>
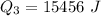
Generally the heat of vaporization of the Ethanol is mathematically represented as
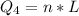
=>
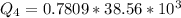
=>
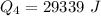
Generally the heat required too raise the Ethanol from its boiling point to
 is mathematically represented as
is mathematically represented as
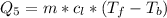
=>
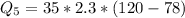
=>
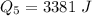
Generally the total heat required is
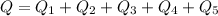
=>
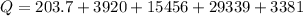
=>