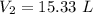Answer:
The volume is
Step-by-step explanation:
From the question we are told that
The number of moles of the gas is
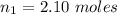
The volume of the gas is
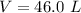
The of moles of the gas that leaked is
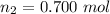
Generally from the ideal gas law
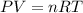
Generally at constant pressure and temperature
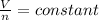
So
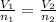
=>
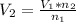
=>
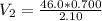
=>