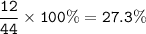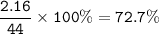%C = 27.3%
%O=72.7%
Further explanation
Proust states the Comparative Law that compounds are formed from elements with the same Mass Comparison, so that compounds have a fixed composition of elements
The empirical formula is the mole ratio of compounds forming elements.
MW CO₂ : 44 g/mol
Ar C = 12 g/mol
Ar O = 16