Answer:
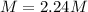
Step-by-step explanation:
Hello!
In this case, since the molarity and volume of the solutions are given, we are able to compute the moles of chloride ions as they are present in NaCl only:
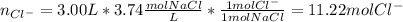
Next, since the mixing of solution A and B lead to a volume of 5.00 L, the concentration of chloride ions turns out:
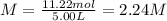
Best regards!