Answer:
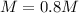
Step-by-step explanation:
Hello!
In this case, since the presence of silver ions is in AgNO3 only, we can compute their moles by using the volume and concentration of the corresponding salt:
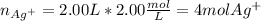
Next, since the total volume of solution C is 5.00 L, the required concentration of silver ions turn out:
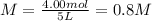
Best regards!