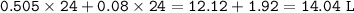The total volume of gas present (in L) : 14.04 L
Further explanation
Reaction(balanced) :
C₂H₅OH(g) + 3O₂(g) → 2CO₂(g) + 3H₂O(l)
mol C₂H₅OH(MW=46,07 g/mol) :
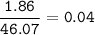
mol O₂(MW=32 g/mol) :
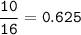
Limiting reactant :
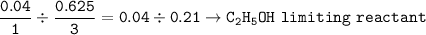
mol CO₂ =
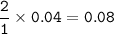
mol O₂(unreacted) :
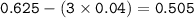
Conditions at T 25 ° C and P 1 atm are stated by RTP (Room Temperature and Pressure). Vm(molar volume) in this condition = 24 liters / mol
Total volume of gas :
volume O₂+volume CO₂ =