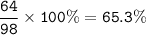The percent of SO₂ in H₂SO₄ : 65.3%
Further explanation
Proust states the Comparative Law that compounds are formed from elements with the same Mass Comparison so that compounds have a fixed composition of elements
%Mass A in AxBy =
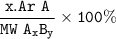
For SO₂ in H₂SO₄ :
MW SO₂ = 32 + 2.16=64 g/mol
MW H₂SO₄ =2.1+32+4.16=98 g/mol
The percent of SO₂ :