To Find :
The mass, in grams, of
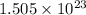 molecules of carbon disulfide (CS₂).
molecules of carbon disulfide (CS₂).
Solution :
Molecular mass of CS₂ = 76 gm/mol .
It means that mass of
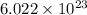 molecules of CS₂ is 76 gm/mol.
molecules of CS₂ is 76 gm/mol.
Let, mass of
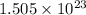 molecules of carbon disulfide (CS₂) is x.
molecules of carbon disulfide (CS₂) is x.
So,
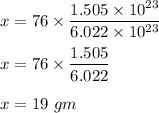
Therefore, the mass in gram is 19 gm.