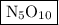Answer:
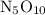
Step-by-step explanation:
- The atomic mass of nitrogen is 14.0067 g/mol.
- The atomic mass of oxygen is 15.9994 g/mol.
This means the molecular mass of nitrogen dioxide is
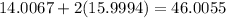 g/mol.
g/mol.
Dividing this molecular mass of the compound we need to find, we get that
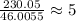 .
.
So, the final answer is