Given :
Volume of stock solution,
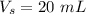 .
.
Volume of diluted solution,
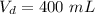 .
.
Molarity of diluted solution,
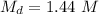 .
.
To Find :
The concentration of the stock solution.
Solution :
We know, dilution factor is given by :
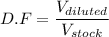 ....1)
....1)
and
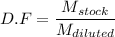 ....2)
....2)
Putting volume in above equation, we get :
D.F = 400/20
D.F = 20
Putting value of D.F in equation 2) we get :
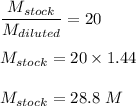
The concentration of stock solution is nearest to 30 M.
Therefore, the correct option is 1) 30 M.