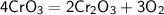Question -:
Balance the following equation.
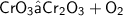
Explanation -:
The law of conservation of mass was put forward by Antoine Laurent Lavoisier. Law of conservation of mass states that matter is neither created nor destroyed during a chemical reaction. This means that there is no change in mass during a chemical reaction.
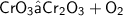
Word equation
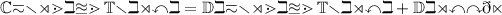
Reactant :
Chromium Trioxide = CrO3
Product :
Dichromium Trioxide + Dioxygen = Cr2O3 + O2
Balanced equation.