Answer:
pH = 4.164
Step-by-step explanation:
The first process is to find the initial moles for the base (B) & the acid (HA)
i.e.
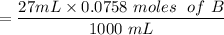
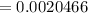
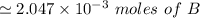
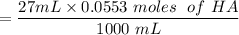
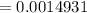
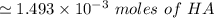
The acid with base reaction is expressed as;
HA + B → A⁻ + HB⁺
to 1.493 × 10⁻³ 2.047 × 10⁻³ - -
- 1.493 × 10⁻³ 1.493 × 10⁻³ 1.493 × 10⁻³ 1.493 × 10⁻³
0 5.54 × 10⁻⁴ 1.493 × 10⁻³ 1.493 × 10⁻³
From observation; both the acid & base weak
Given that:
The pKa for base = 4.594
The pKa for acid = 3.235
Recall that;
pKa = -log Ka
So; Ka =
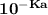
By applying this:
For Base; Ka =
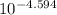 = 2.5468 × 10⁻⁵
= 2.5468 × 10⁻⁵
For Acid: Ka =
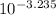 = 5.821 × 10⁻⁴
= 5.821 × 10⁻⁴
After the reaction; we have the base with its conjugate acid & conjugate base of acid; Thus, since the conjugate acid of the base possesses a higher value of K, it is likely it would be the one to define the pH of the solution.
By analyzing the system, we have:
HB⁺ + H₂O ↔ B + H₃O⁺
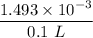
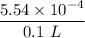
to 0.01493 M 0.00554 M
- x x x
0.01493 - x 0.00554 - x x
Thus;
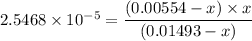
Using the common ion effect;
0.00554 - x
 0.00554 &
0.00554 &
0.01493 - x
 0.01493
0.01493
∴
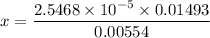
x = [H₃O⁺] = 6.8635 × 10⁻⁵
∴
pH = -log(6.8635 × 10⁻⁵)
pH = 4.164