Answer:
92.4%
Step-by-step explanation:
The volume per cubic meter of sodium preoccupy by the sodium ions is
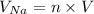
where;
volume (V) of each Na atom =
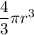
Radius of each ion = 97.3 pm = 97.3 × 10⁻¹²
no.of atoms in the sample n = mass of the sample / ( molar mass / NA)
mass of the sample per cubic metre = 911 kg/m³
∴
![V = \bigg [ (4)/(3) \pi r^3 \bigg ] \bigg [(MN_A)/(m) \bigg ]](https://img.qammunity.org/2021/formulas/chemistry/college/npprsyje2mm3tdcq5wehvyh0ul0h80iw3a.png)
![V = \bigg [ (4)/(3) \pi (97.3 * 10^(-12) )^3 \bigg ] \bigg [( (911 kg/m^3 (m^3))(6.023* 10^(23)))/(27.7 *10^(-3)\ kg/mol ) \bigg ]](https://img.qammunity.org/2021/formulas/chemistry/college/9y5ijoafsu2qmv2phiwwnvaedmt5brg4ck.png)
V = 0.07643 m³
The fraction of available conduction electrons are;
= (1 - V)
= 1 - 0.07643
= 0.92357
≅ 92.4%