Answer:
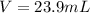
Step-by-step explanation:
Hello!
In this case for the solution you are given, we first use the mass to compute the moles of CuNO3:
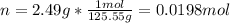
Next, knowing that the molarity has units of moles over liters, we can solve for volume as follows:
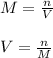
By plugging in the moles and molarity, we obtain:
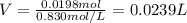
Which in mL is:
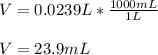
Best regards!