Answer:
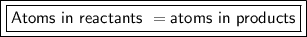
Step-by-step explanation:
The Law of Conservation of Mass states that mass cannot be created or destroyed.
In a chemical reaction, this means the mass of the reactants (substances going in and being changed) must equal the mass of the products (new substances from the reaction).
In addition, the amount of atoms must stay the same. The atoms can and will reconfigure to form different compounds, but the number of atoms stays the same.