Answer:
0.87 atm
Step-by-step explanation:
The new pressure can be found by using the formula for Boyle's law which is
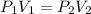
Since we are finding the new pressure
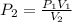
From the question
2.47 L = 2470 mL
We have
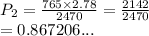
We have the final answer as
0.87 atm
Hope this helps you