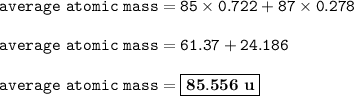The average atomic mass of Rubidium : 85.556 u
Further explanation
Isotopes are atoms that have the same atomic number but different mass numbers
Each isotope has an abundance which is usually expressed as a percentage
The average relative mass of atoms is obtained by adding the product of the percentage and mass of each isotope
Can be formulated:
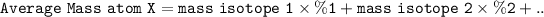
The abundance of 85 Rb is 72.2% and the abundance of 87 Rb is 27.8%, so
mass isotope 1=85, %abundance=72.2
mass isotope 2=87, %abundance=27.8
The averages atomic mass of Rubidium :