Answer: 1169.298 L
Explanation: In order to go from grams to liters, you must find the moles first using the molar mass, 62.068 g/mol (found via google search). I will assume the ethylene glycol is in gas form so we can use the 22.4 L per mol conversion.
Using dimensional analysis, starting with the kg, we must convert to grams, then moles, then liters.
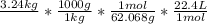
Multiply the top individually, then the bottom individually.
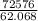
Divide out.
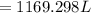
In the end, we get 1169.298 L of ethylene glycol.