Answer: The ratio of carbon to bromine atoms in the molecule is 3:1
Step-by-step explanation:
Compound is a pure substance which is made from atoms of different elements combined together in a fixed ratio by mass. It can be decomposed into simpler constituents using chemical reactions.
Chemical formula shows the elements in a compound and the relative proportions of those elements.
The chemical formula given for the compound is
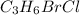 which means the ratio of carbon to bromine atoms is 3: 1.
which means the ratio of carbon to bromine atoms is 3: 1.
Thus the ratio of carbon to bromine atoms in the molecule is 3:1