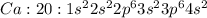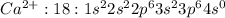Answer: 4s orbital contains the electron that calcium loses to form an ion
Step-by-step explanation:
Electronic configuration represents the total number of electrons that a neutral element contains. The electrons are filled according to Afbau's rule in order of increasing energies.
Calcium with atomic number 20 has 20 electrons and has 2 valence electrons which it can lose to for stable
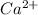 ion. The valence elctrons are present in valence shell i.e. 4s.
ion. The valence elctrons are present in valence shell i.e. 4s.