The frequency : a) 7.5 x 10¹⁴ /s
Further explanation
Radiation energy is absorbed by photons
The energy in one photon can be formulated as
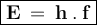
Where
h = Planck's constant (6,626.10⁻³⁴ Js)
f = Frequency of electromagnetic waves (/s or Hz)
f = c / λ
c = speed of light
= 3.10⁸ m/s
λ = wavelength
The wavelength(λ) of purple light is 400 x 10⁻⁹ m, so the frequency :
