Answer:
No of Moles in excess at the end of the reaction is 0.25 moles
Step-by-step explanation:
AgNO3 + Mg3P2 → Ag3P + Mg(NO3)2
Balancing the equation we get
6AgNO3 + Mg3P2 → 2Ag3P + 3Mg(NO3)2
6 moles of AgNO3 needs 1 mole of Mg3P2
using unitary method
AgNO3 =
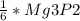
1.5 AgNO3 =
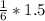
= 1/4 = 0.25moles of Mg3P2
So 1.5 Moles of AgNO3 requires 0.25Mg3P2 for complete reaction but we have 0.5Moles of Mg3P2 available Therefore Mg3P2 is in excess
No of Moles in excess at the end of the reaction = 0.5 - 0.25 = 0.25moles