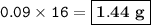Mass of element P = 1.44 g
Further explanation
Proust stated the Comparative Law that compounds are formed from elements with the same Mass Comparison so that the compound has a fixed composition of elements
In the same compound, although from different sources and formed by different processes, it will still have the same composition/comparison
MW Q₂P₃ = 2.27 + 3.16=102 g/mol
Mass Q₂P₃ (MW=102 g/mol) :
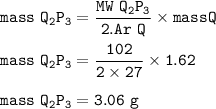
Mass P (Ar=16) :
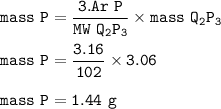
or you can solve it with mol :
Reaction
3P + 2Q ⇒ Q₂P₃
mol Q :
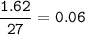
mol P :
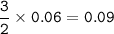
mass P :