Answer:
0.0125mol/dm³
Step-by-step explanation:
Given parameters:
Mass of carbon dioxide = 0.22g
Volume of water = 400cm³
Unknown:
Concentration in mol/dm³ = ?
Solution:
Concentration is the amount of solute dissolved in a solvent.
The formula is expressed as;
Concentration =
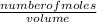
Number of moles =

Molar mass of CO₂ = 12 + 3(16) = 44g/mol
Number of moles =
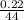 = 0.0005mol
= 0.0005mol
Now,
1000cm³ = 1dm³
400cm³ =
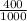 = 0.4dm³
= 0.4dm³
Insert the parameters and solve;
Concentration =
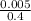 = 0.0125mol/dm³
= 0.0125mol/dm³