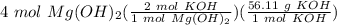Answer:
A.
General Formulas and Concepts:
Chemistry - Stoichiometry
- Using Dimensional Analysis
Step-by-step explanation:
Step 1: Define
Given: 4 mol Mg(OH)₂
RxN: MgCl₂ + 2KOH → Mg(OH)₂ + 2KCl
Step 2: Identify Conversions
Molar Mass of K - 39.10 g/mol
Molar mass of O - 16.00 g/mol
Molar mass of H - 1.01 g/mol
Molar Mass of KOH - 39.10 + 16.00 + 1.01 = 56.11 g/mol
Step 3: Stoichiometry