Answer:
0.36mol
Step-by-step explanation:
Given parameters:
Volume of NaCl = 0.4L
Molarity = 0.9M
Unknown:
Number of moles = ?
Solution:
To find the number of moles, the molarity of a solution is the amount of solute contained in a solution.
Molarity =
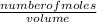
Number of moles = molarity x volume
Insert the parameters and solve;
Number of moles = 0.4 x 0.9 = 0.36mol