Answer:
66 gallons
Explanation:
Given that initially 160 gallons of a solution containing 12% salt.
So, the initial amount of salt = 12% of 160 = 12/100 x 160 = 19.2 gallons.
Let x gallons of water get evaporated so that the remaining solution contains 20% salt.
The remaining amount of solution = 160-x gallons.
As the initial amount of salt is still in the solution which is equal to 20% of the remaining solution, so
19.2 = 20% of (160-x)
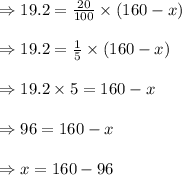
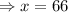 gallons
gallons
Hence, 66 gallons of water must be evaporated to get a 20% salt solution.