Answer:
5.595 g Fe
General Formulas and Concepts:
Chemistry - Stoichiometry
- Reading a Periodic Table
- Using Dimensional Analysis
Step-by-step explanation:
Step 1: Define
16.25 g FeCl₃ (Iron III Chloride)
RxN: 2Fe + 3Cl₂ → 2FeCl₃
Step 2: Identify Conversions
Molar Mass of Fe - 55.85 g/mol
Molar Mass of Cl - 35.45 g/mol
Molar Mass of FeCl₃ - 55.85 + 3(35.45) = 162.2 g/mol
Step 3: Stoichiometry
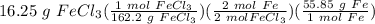 = 5.59533 g Fe
= 5.59533 g Fe
Step 4: Check
We are given 4 sig figs. Follow sig fig rules and round.
5.59533 g Fe ≈ 5.595 g Fe