Answer:
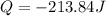
Step-by-step explanation:
Hello!
In this case, since energy involved when a substance undergoes a temperature change due to heat addition or removal is computed as shown below:
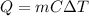
As the aluminum has a specific heat of 0.900 J/g°C and 14.4 g underwent such temperature decrease, the energy change is:
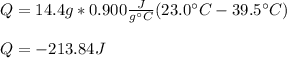
Which is an outlet energy flow.
Best regards!