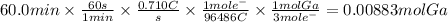Answer:
0.00883 mol
Step-by-step explanation:
Let's consider the reduction reaction of gallium.
Ga³⁺(aq) + 3 e⁻ ⇒ Ga(s)
We can establish the following relationships:
- 1 mole of electrons have a charge of 96486 C (Faraday's constant)
- When 3 moles of electrons circulate, 1 mole of Ga is deposited
The amount of Ga deposited using a current of 0.710 A that flows for 60.0 min is: