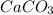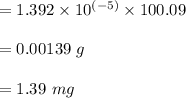Answer:
The answer is "
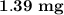 ".
".
Step-by-step explanation:
Please find the complete question in the attached file.
Total Moles of
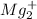 = moles of
= moles of
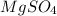
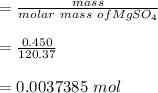
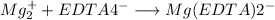
EDTA mol in 37.6 mL of solution = 50.0 mL of
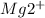
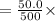 total moles of
total moles of
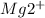
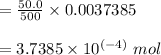
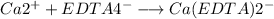
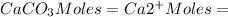 EDTA moles in a solution of 1.40 mL
EDTA moles in a solution of 1.40 mL
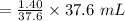 the solution of EDTA moles.
the solution of EDTA moles.
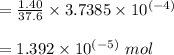
Mass of
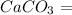 mole
mole
 the molar mass of
the molar mass of