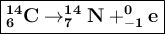The decay reaction of Carbon-14 which releases Beta particles, resulting in the element Nitrogen-14
Further explanation
Radioactivity is the process of unstable isotopes to stable isotopes by decay, by emitting certain particles,
- alpha α particles ₂He⁴
- beta β ₋₁e⁰ particles
- gamma particles ₀γ⁰
- positron particles ₁e⁰
- neutron ₀n¹
Carbon-14 emits beta β ₋₁e⁰ particles , so the atomic number increases by 1, the mass number remains the same
Reaction