Answer:
Uranium-238 has a much longer half-life of 4.5 billion years versus Carbon-14's 5,730 years.
Step-by-step explanation:
Radioactive Carbon-14 or
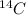 is typically formed in the upper atmosphere. In plants, it gets incorporated into organic molecules through photosynthesis, and this material is ingested by consumers.
is typically formed in the upper atmosphere. In plants, it gets incorporated into organic molecules through photosynthesis, and this material is ingested by consumers.
It is used for the process of radiocarbon dating of fossils and formations. Radioactive isotope dating is an effective means of identification due to the unstable nature of the isotope used and the predictable rate of decay.
Over time they decay to give off an electron, in an effort to become a more stable element. For instance,
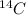 has a relatively short half-life and half the in a sample becomes Nitrogen over a few thousand years (5,730 years).
has a relatively short half-life and half the in a sample becomes Nitrogen over a few thousand years (5,730 years).
However, for older samples, other isotopes are used because their long half-life may range between a million to billions of years. Uranium-238, with a half-life of 4.5 billion years is often used in geochronology; uranium-lead dating is suitable for older specimens.