Answer:
0.00735°C
Step-by-step explanation:
By seeing the question, we can see the elevation in boiling point with addition of BaCl₂ in water
⠀
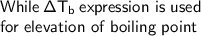
⠀
⠀
The elevation in boiling point is a phenomenon in which there is increase in boiling point in solution, when the particular type of solute is added to pure solvent.
⠀
⠀
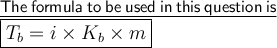
⠀
⠀
Where 'i' is van't hoff factor which represents the ratio of observed osmotic pressure and the value to be expected.
and 'i' is 3 (as given in the question)
⠀
'Kb' is molal boiling point constant. And it's value is 0.51°C/mol(given in question)
⠀
'm' represent the molality of solution. Molatity is no. of moles of solution present in 1kg of solution.
⠀
⠀
To find molality, we have to divide no. of moles of solute by weight of solution
⠀
While first we need to no. of moles

⠀
⠀
Now, we will find molality
⠀
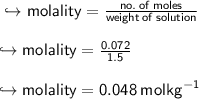
⠀
⠀

⠀
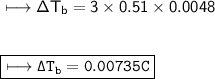
⠀
⠀
⠀
Henceforth, the change in boiling point is 0.00735°C.