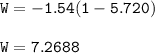The internal energy of a system : 21.9 kJ
Further explanation
The laws of thermodynamics 1 state that energy can be changed but cannot be destroyed or created
The equation is:
ΔU=Q+W
Energy owned by the system is expressed as internal energy (U)
The sign rules for heat and work are set as follows:
• The system receives heat, Q +
• The system releases heat, Q -
• The system does work, W -
• the system accepts work, W +
The system absorbs 14.73 kJ⇒Q=+14.kJ
The system compressed⇒work done on the gas⇒W=+
W=-PΔV