Mass of X produced : D. 4.6 g
Further explanation
Reaction
Decomposition of Magnesium nitrate into Magnesium oxide, Oxygen, and Nitrogen oxides
2 Mg(NO₃)₂ → 2 MgO + 4 NO₂ + O₂
mol Magnesium nitrate-Mg(NO₃)₂(MW=148,3 g/mol) :
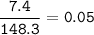
mol ratio Mg(NO₃)₂ : NO₂ = 2 : 4, so mol NO₂ :
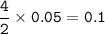
mass NO₂(MW=46 g/mol) :
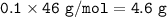
.