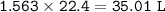The volume of the gas at STP = 35.01 L
Further explanation
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure).
In general, the gas equation can be written
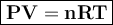
where
P = pressure, atm
V = volume, liter
n = number of moles
R = gas constant = 0.08206 L.atm / mol K
T = temperature, Kelvin
V=17.4 L
T = 23 + 273 = 296 K
P = 2.18 atm

The volume of the gas occupy at STP :