Answer:
3.56 moles
Step-by-step explanation:
The number of moles of glucose can be found by using the formula
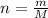
where
n is the number of moles
M is the molar mass
m is the mass of the substance
First we must find the molar mass of glucose
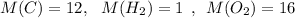
The molar mass of glucose is
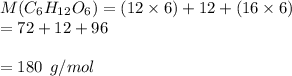
mass = 640 g
So we have
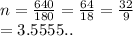
We have the final answer as
3.56 moles to 2 decimal places
Hope this helps you