Answer:
To answer the question, we correctly fill the attached screenshot as follows;
- 3H₂ + N₂ → 2NH₃
- The molar mass of H₂ = 2 g/mol
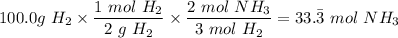
The molar mass of N₂ = 28 g/mol
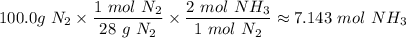
A. Therefore, the excess reactant is hydrogen gas H₂ because it makes the most amount of ammonia, NH₃ (33.
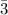 moles of NH₃)
moles of NH₃)
B. The limiting reactant in nitrogen, N₂, because it is the reactant that makes the least amount of the ammonia, NH₃ (approximately 7.143 mol NH₃)
C. The theoretical yield of ammonia, is the maximum amount of ammonium that can be produced from the reaction between the 100 g of hydrogen gas, H₂, and 100 g of nitrogen gas, N₂ which is given by the amount of ammonia produced by the limiting reactant which is approximately 7.143 mole of NH₃
Step-by-step explanation: